Rank the Structures Shown From Most to Least Stable.
100 5 ratings Transcribed image text. II III IV 1 C.
Meanwhile the least stable structures are known as the minor contributors.

. View the full answer. The most stable structures are known as the major contributors. Show transcribed image text.
Benzylic carbocations are so stable because they have not one not two but a total of 4 resonance structures. I need help ranking the following from most stable to least stable. 05 pts A 1 B 2 C 3 D 4 CH2-CH2-CH3 3 Predict the major.
Solution for Rank the following from most to least stable. Which of the structures shown depicts the most stable carbocation intermediate formed in the hydrohalogenation reaction shown. If the specific rotation for this compound is known to be 100 what would be the specific rotation for the stereoisomer shown at the right.
05 pts 2 Which position would react at the fastest rate with a bromine radical. Rank the structures shown crom most to least stable. We review their content and use your feedback to keep the quality high.
Chemistry questions and answers. What is important as well is that not all the resonance structures are equally stableIn fact the most stable resonance form is the resonance hybrid since it delocalizes the electron density over a. - III IV A.
Rank the resonance structures below from most to least important. Rank the following base pairs according to their stability. Rank the structures shown from most to least stable.
The least stable highest energy conformation will have an eclipsed conformation. Rank them according to decreasing atomic number. Rank the following carbocations a -c in order of increasing stability from least stable to most stable.
The stability order of given alkenes are Option E- IIIIII Reason. For each of the following draw the best most stable and worst least stable Newman projection relative to the bond indicated in each question. Rank from most to least stable.
Draw the condense structure for the following carboxylic acids. Hence the correct option is A. What is the expected major products of HCl.
C a b. IVv III most stable least stable. Rank the following base pairs according to their stability.
Question 3 Rank the structures shown from most to least stable. Often one of the resonance structures will be more stable so it will contribute to the hybrid more than the others. The most stable conformations will be staggered conformations with the largest groups ANTI to each other.
Review Benzene resonance in this video This shares the burden of charge over 4 different atoms making it the MOST stable carbocation. Rank the structures shown from most to least stable. - CH32CCH2CH3 with dot over the C.
With the most Hs the least substitutent carbon and the X will add. Experts are tested by Chegg as specialists in their subject area. Previous question Next question.
Two molecules reacting to form a single molecule. - CH32CCHCH2 with dot over the CH2. ОН H 도 도 - II III A B I D E.
1Il IV 11 III IV III IV 11 V DIV. To rank items as equivalent overlap them. Correct option is A Among the given resonance structures the least stable resonance structure is given by option A as there is minimum charge separation between the like charges which increase and decrease the stability.
IV 2Rank the conformers of 1 2 4-trimethylcyclohexane in order of decreasing stability putting the most stable first. Rank from most to least stable. 3 2 4 1.
14 Inductive Effects section 21. The dots represent the primary secondary and tertiary radicals. - CH32CHCH2CH2 with dot over the CH2.
Answer to Solved Rank the structures shown from most to least stable. Rank the following aromatics in order of decreasing reactivity toward electrophilic aromatic substitution Most reactive least reactive. Lone pairs are not explicitly shown think about the number of lone pairs implied by the bond line structures.
What is the alternate chair conformation of the following compound. The Double Helix - Hydrogen bonding and stability. The structure of Crestor rosuvastatin a medication used to reduce cholesterol is shown below.
Solve any question of Organic Chemistry - Some Basic. That are possible the more stable the carbocation. I have found out that the first one is thymine-adenine pair and the second one is.
1 Rank the following radicals in order of decreasing stability I most stable. 1 II OT Il IN O III II 1 O II III 1 O II 1 III O I Ill 11. I II III IV B.
Draw a mechanism for the addition of H2O in the presence of H2SO4 to 2-butyne shown below. Rank the structures shown from most to least stable. Remember resonance structures have the same placement of atoms meaning that they represent the same compound and only the arrangement of electrons is different.
II III OI 111 O III 11 1 O 11 III 1 O 111. This is the best answer based on feedback and ratings. Rank the structures shown from most to least stable.
To rank items as equivalent overlap them. Use the octet rule and electronegativity. Shifting of electrons in a s-bond in response to the electronegativity of a nearby atom or group.
Solution for Rank the structures shown from most to least stable. A 100 B. If two structures are equal they will contribute equally Resonance Stability Rules.
To find out which resonance structure is the most stable there are five main rules to follow. - CH32CHCHCH3 with dot over the 2nd CH. Which of the structures shown depicts the most stable carbocation intermediate formed in the hydrohalogenation reaction shown.
We review their content and use your feedback to keep the quality high. Some professors will rank a primary benzylic carbocation under or near a tertiary carbocation. According to saytzeff Rule.
III IV.
Resonance Structures 4 Rules On How To Evaluate Them With Practice
Cyclohexane Chair Conformation Stability Which One Is Lower Energy

Resonance Structures 4 Rules On How To Evaluate Them With Practice Organic Chemistry Chemistry Help Chemistry Worksheets
Cyclohexane Chair Conformation Stability Which One Is Lower Energy

How To Choose The More Stable Resonance Structure Chemistry Steps
Cyclohexane Chair Conformation Stability Which One Is Lower Energy
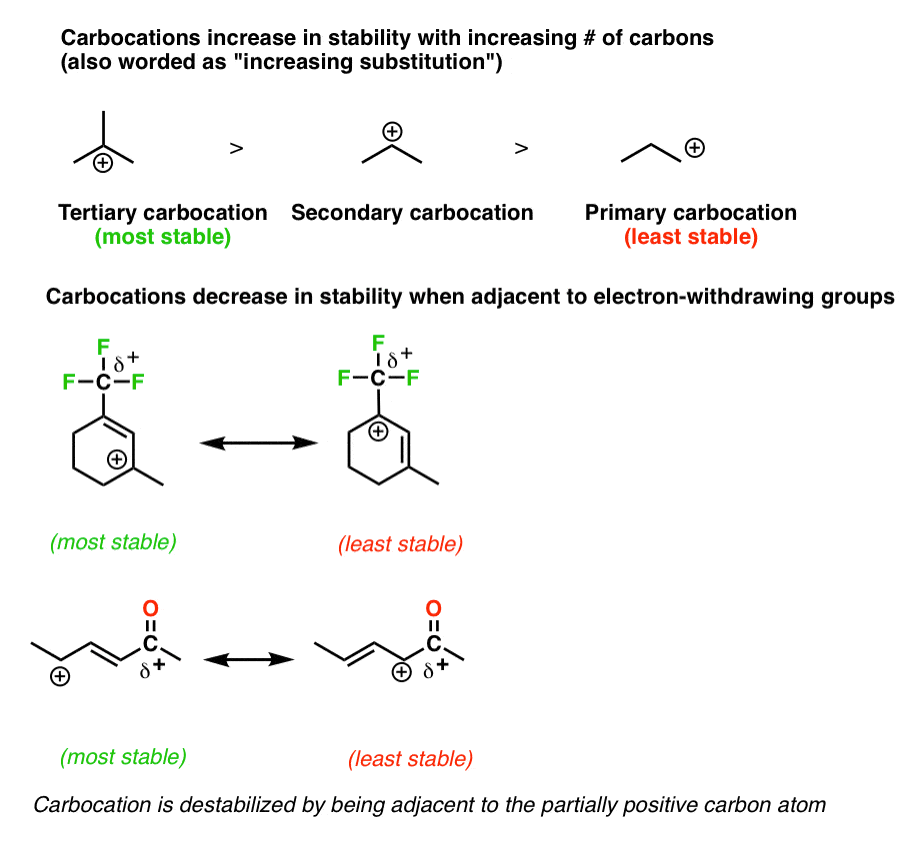
Resonance Structures 4 Rules On How To Evaluate Them With Practice
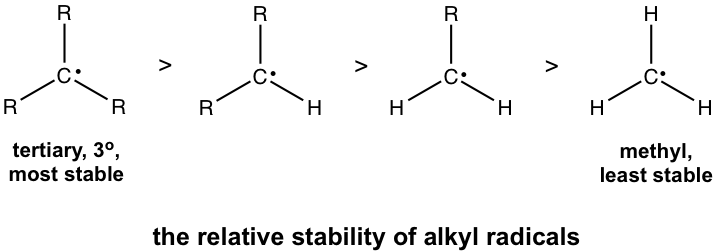
9 3 Stability Of Alkyl Radicals Organic Chemistry I

Page Firing Alpha Metagaming Ceh Pen Belltest Ranking Preserves Alphas Better To Put Me In My Womens Bell Curve Teaching Middle School Firsties

How To Choose The More Stable Resonance Structure Chemistry Steps

Cyclohexane Chair Conformation Stability Which One Is Lower Energy
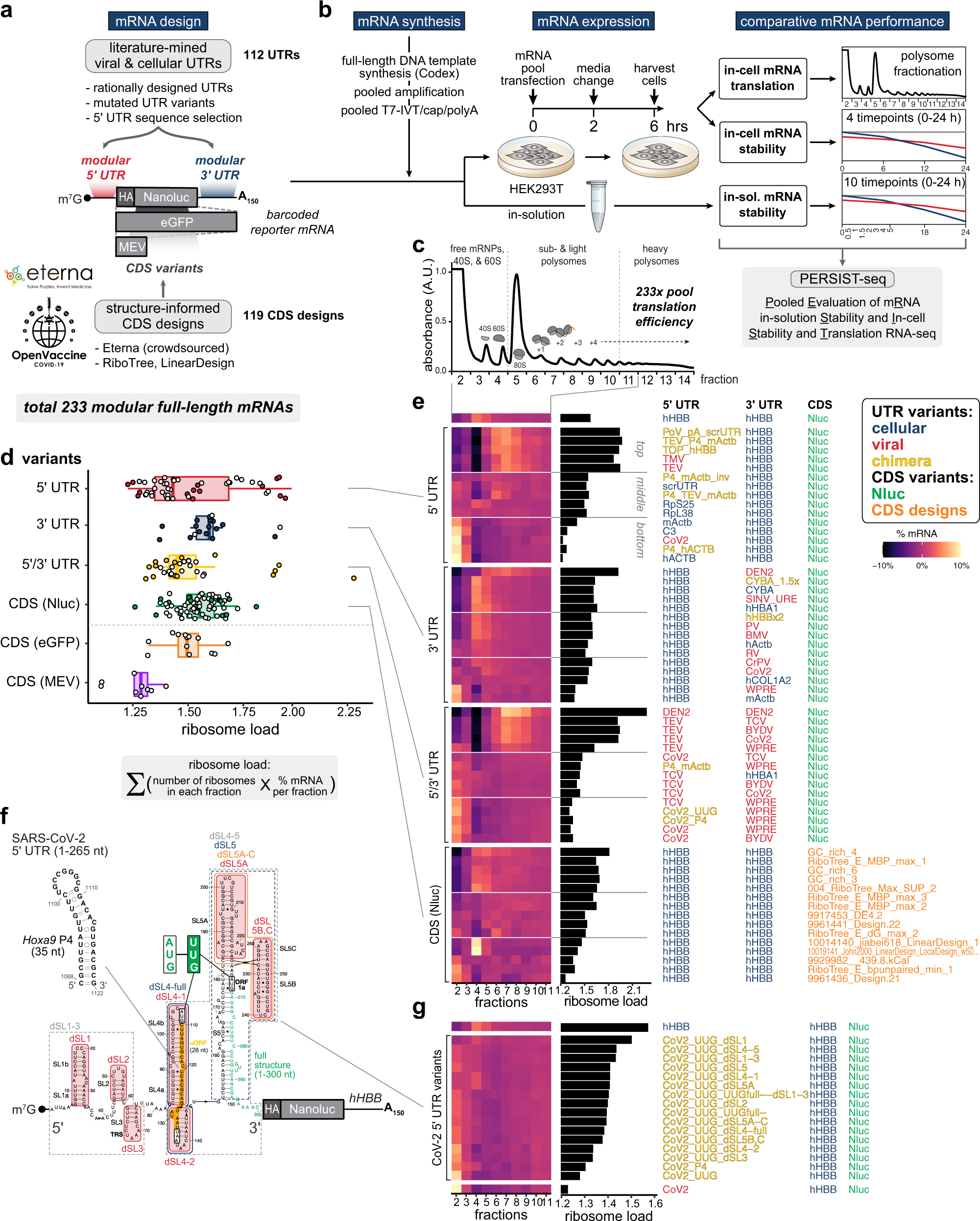
Combinatorial Optimization Of Mrna Structure Stability And Translation For Rna Based Therapeutics Nature Communications

How To Choose The More Stable Resonance Structure Chemistry Steps

How To Choose The More Stable Resonance Structure Chemistry Steps

How To Choose The More Stable Resonance Structure Chemistry Steps

The Pikler Triangle By Rad Children S Furniture Bois Dur Meuble Bebe Mobilier De Salon

3 Factors That Stabilize Carbocations Master Organic Chemistry Organic Chemistry Organic Chemistry Reactions Study Chemistry

Metastable Structure In Electrode Material Of Lithium Ion Battery Chemistry Lithium Ion Batteries Material

Comments
Post a Comment